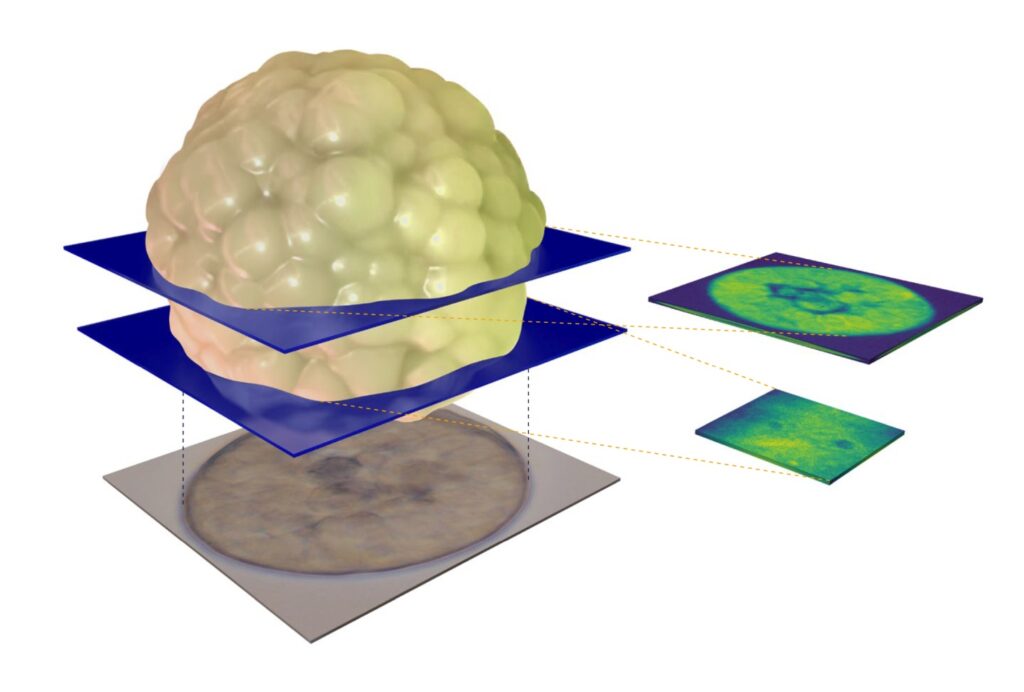
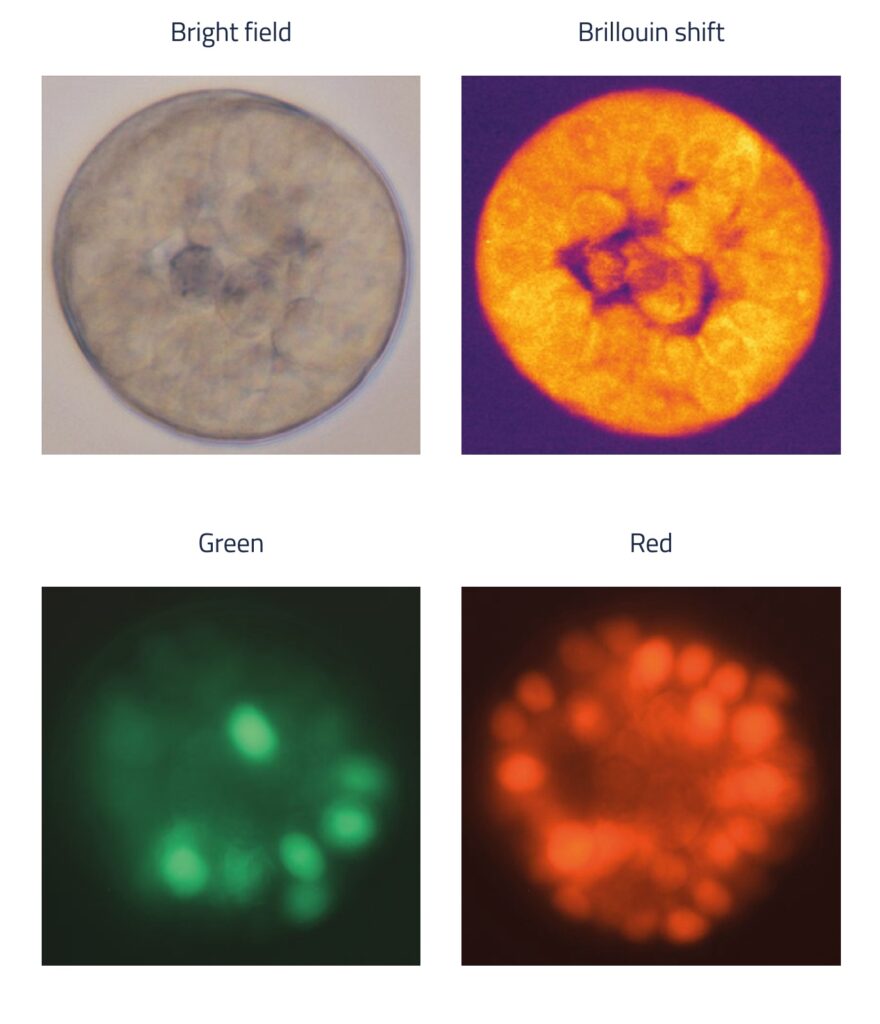
MCF-7 Cancer Organoids investigated by Brillouin Microscopy
Cancer cells often exhibit altered biomechanical properties compared to healthy cells. These mechanical changes can provide insight into tumor aggressiveness, as studies have shown that increased cell deformability may correlate with a higher metastatic potential.
Traditionally, the mechanical properties of cancer cells have been studied using techniques that require isolating single cells from cultures or tissues. However, these methods do not capture how cancer cells behave in their natural 3D environment. In contrast, Brillouin microscopy provides a non-invasive approach to studying cancer cell mechanics in physiologically more relevant conditions, such as 3D tumor models or small tissue fragments.
Recent research from the Taubenberger group at Dresden University of Technology has demonstrated the potential of Brillouin microscopy for characterizing the mechanical properties of 3D in vitro tumor models. In this study, the researchers analyzed MCF-7 breast cancer cells forming tumor spheroids within an ECM-mimicking hydrogel. MCF-7 cells are widely used in breast cancer research because they closely resemble the behavior of estrogen-responsive tumors.
For this study, MCF-7 cells were cultured in a hydrogel for 12 days. Since the cells expressed a cell cycle reporter (FUCCI), their progression through the cell cycle could be tracked in real time. The researchers observed that in compliant hydrogels (1–2 kPa), some spheroids developed a central lumen—a structural feature of mammary morphogenesis that is typically lost in tumorigenesis. Imaging at multiple depths, both through the center and along the outer edge, provided a detailed 3D representation of a live organoid.
Brillouin microscopy allowed the team to analyze the biophysical properties of both cells and the luminal space, regions that are difficult to assess with conventional techniques. With subcellular resolution, they could distinguish the mechanical properties of cellular organelles, including nuclei and nucleoli.
Because Brillouin microscopy works on live samples, the researchers could monitor structural changes over time. The FUCCI reporter enabled them to track cell cycle progression while simultaneously measuring how biophysical properties evolved at different stages. Additionally, the researchers observed how cells proliferate and organize in response to mechanical and biochemical signals from their microenvironment.
A deeper understanding of these 3D tumor models, particularly their mechanical properties and structural organization, is crucial for developing new targeted therapies. By providing non-invasive, real-time insights into tumor biomechanics, Brillouin microscopy is helping advance personalized medicine and the development of precision cancer treatments.